Infographic
Accelerate Innovation in Medical Device Development
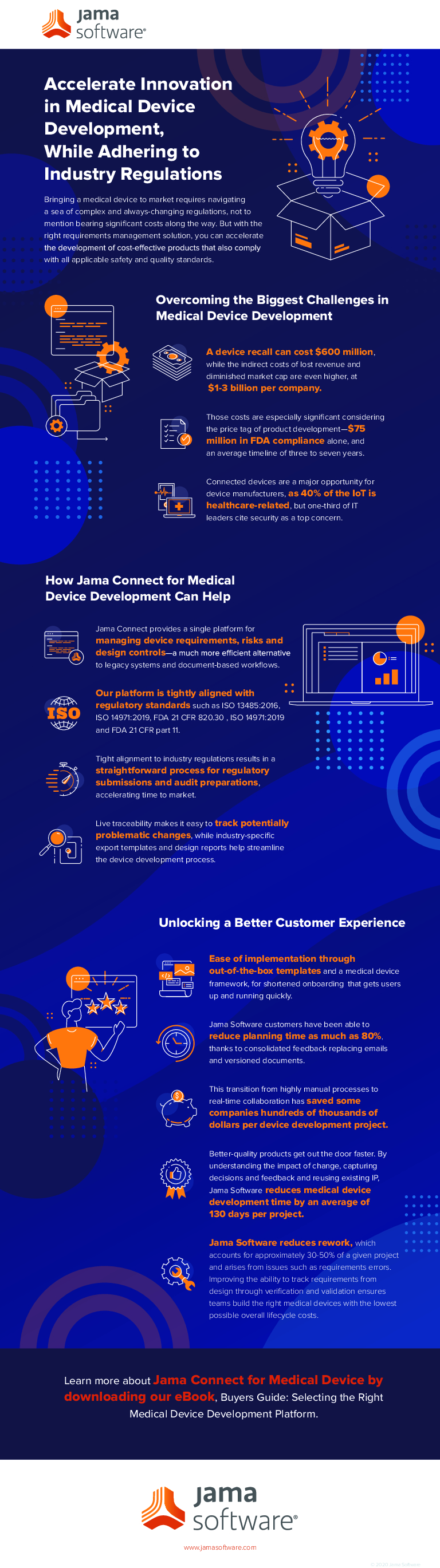
Bringing a medical device to market requires navigating a sea of complex and ever-changing regulations, not to mention bearing significant costs along the way. In this infographic we share how, with the right requirements management solution, you can accelerate the development of cost-effective products that also comply with both safety and quality standards. You’ll learn:
- How to overcome the biggest challenges in medical device development
- The ways Jama Connect for Medical Device Development can help
- Keys to unlocking a better customer experience